God’s Gift of Metals: Crafted for Life
Formed to Be Inhabited
Abstract
This is the final of four articles relating to how the earth was formed to be inhabited. In this article we’ll look briefly at some of the many metals that permit life and make the earth habitable. When we think of metals, we typically think of hard, often-shiny elements like gold, silver, or chromium. Most of the metals we’ll see here are in groups 1A and 2A of the Periodic Table, the alkali metals and alkaline earth metals, respectively. These metals are shiny, soft and malleable, and have low melting points. They are fairly reactive and hence are never found in a pure state, but only in combination with other elements. They tend to give up electrons, easily becoming oxidized, so we often indicate the oxidation state of the element with a superscript (e.g., Ca2+).
Earth—Formed to Be Inhabited
- God’s Flawless Design of Water Makes Life Possible
- Carbon: God’s Building Blocks for Life
- Proportionally Perfect for Life: O2, CO2, N2
- God’s Gift of Metals: Crafted for Life
At-a-Glance
- Iron is virtually indispensable to life. It participates in many life processes such as energy generation by the cell, oxygen delivery by the blood, and nitrogen fixation by bacteria.
- Magnesium’s electronic configuration makes it most suitable for absorbing certain wavelengths of light so that plants can carry out photosynthesis. It also absorbs those wavelengths that would tend to generate toxic free radicals, which are very harmful to life. Magnesium is often complexed to various molecules where it can act as a stabilizing factor for these molecules. Magnesium plays a vital role in the synthesis of DNA and RNA as well as proteins.
- Molybdenum and cobalt play essential roles in the fixation of gaseous nitrogen into organic compounds by bacteria. Many other life forms, such as humans, depend on nitrogen fixation to meet their nitrogen needs. Cobalt also plays an important role in the synthesis of the amino acid methionine in humans, as well as in the metabolism of certain fatty acids obtained from eating plants.
- Copper is crucial to the enzyme superoxide dismutase, which destroys highly toxic free radicals formed by normal metabolism, and by exposure to certain environmental chemicals and wavelengths of light in the ultraviolet range. It is also utilized, along with iron, by cells in plants and animals to conduct electrons.
- Calcium performs many different roles such as neural transduction across synapses, activation of developmental processes at fertilization, as a second messenger helping cells respond to their environment, as a structural component in bones, and aiding in muscle contraction, to mention a few.
- Members of the alkali metals such as sodium and potassium are used in neural transmission, as well as counterions inside cells. The concentrations of these ions are such that they help regulate protein-protein and protein-nucleic acid (RNA and DNA) interactions.
- Not all metals are designed for use in cells, but many are designed to be used by man in constructing shelters, forms of transportation, and a host of other products that enhance the habitability of the earth.
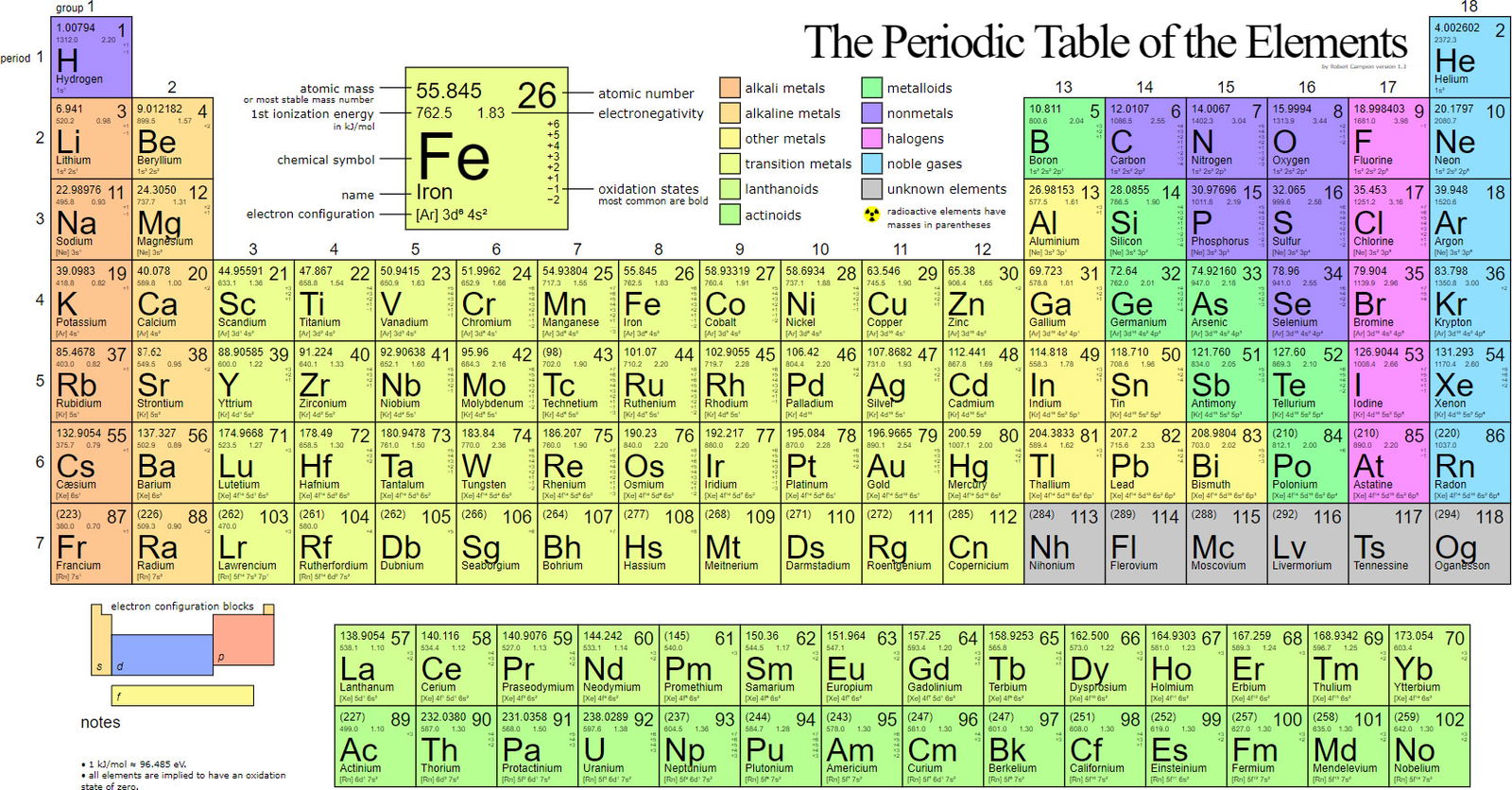
Figure 1 shows the Periodic table of the elements. Metals are indicated by the color legend at the top. Image by Paulmiko, via Wikimedia Commons.
A look at the periodic table of the elements and examination of the elemental make-up of living organisms can quickly confirm Genesis 1 which states that man is formed from the dust of the earth. There are about 92 naturally occurring elements in the periodic table. Approximately 25% of these are considered essential to life. Many are alkali metals such as sodium (Na+), potassium (K+), alkaline earth metals, magnesium (Mg2+), and calcium (Ca2+). Many others are transition metals, such as iron (Fe2+/3+), copper (Cu1+/2+), cobalt (Co2+/3+), and zinc (Zn2+). The use of metals by life is so prevalent and important that, as stated by Robert J. P. Williams of Oxford University, “there is no biology without metal ions.”1 At least a third of all enzymes use one or more metal ions to catalyze their reactions. Metal ions are used to help position substrates in enzyme active sites, to provide electron “sinks,” and/or serve as a source of electrons during catalysis. They function as structural support for many proteins and conduct electrons to oxygen within the electron transport chain in mitochondria for energy production.
Transition Metals
Iron (Fe2+/3+)
The electron configuration of iron makes it more effective than other metals for the reversible binding of oxygen in hemoglobin. This property allows hemoglobin not only to increase the oxygen-carrying capacity of blood, but also to release sufficient quantities of oxygen to the tissues as needed. Perhaps one of the most important roles for iron is its participation in the electron transport chain (ETC) of the mitochondrial inner membrane. The ETC consists of several iron-sulfur complexes, which conduct electrons from reduced molecules ultimately to oxygen to form water. In this process an electrochemical gradient of H+s is generated across the inner membrane. The potential energy in this gradient is used to synthesize the high-energy compound known as adenosine triphosphate, or ATP. While ATP is not the only high-energy compound in the cell, it is the main energy currency for virtually all cells.
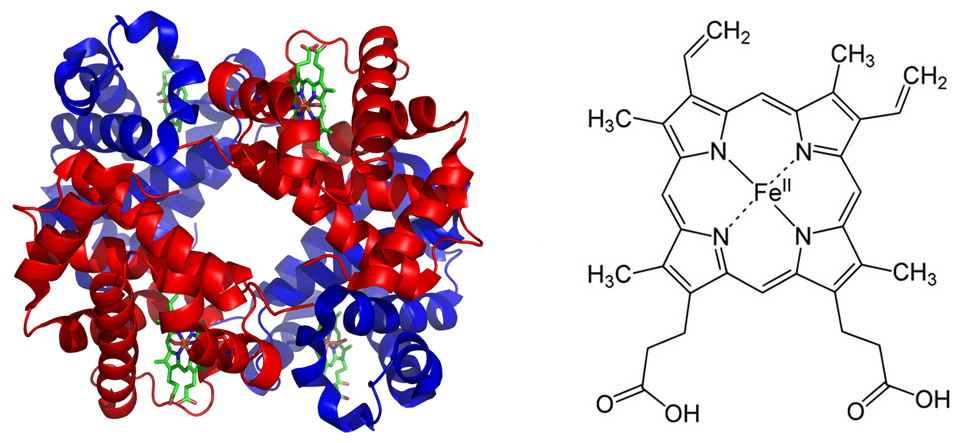
Figure 2 shows the hemoglobin molecule (left) and the porphyrin ring (heme, right) which carries the iron (Fe2+) in the center. Note that there are four heme groups per hemoglobin molecule each with an iron atom. Thus each hemoglobin molecule can carry four oxygen molecules (O2). Image of hemoglobin by PatriciaR, via Wikimedia Commons; image of porphyrin ring by Yikrazuul, via Wikimedia Commons.
Molybdenum (Mo)
Molybdenum has multiple oxidation states from 0 to +6. Together with two other transition metals, iron and cobalt, molybdenum is uniquely configured to serve in the catalytic center of two enzymes, nitrogenase and nitrogen reductase. These two enzymatic activities are essential to all life as these function in the fixation of nitrogen into organic compounds. As stated in the previous article on gases, animals and humans obtain all their nitrogen needs through food. If not for these two specifically designed enzymes, plants, animals, and humans would not be able to make DNA, proteins, and the many other molecules critical for life.
Cobalt (Co2+)
As stated above, Co2+ plays significant roles in nitrogen fixation. However, humans use it for very different purposes. We obtain Co2+ in the form of vitamin B12, or cobalamin. Without this vitamin, and the cobalt component, we could not resynthesize the amino acid methionine from what we ingest and other compounds. When proteins are made by the cell, the first amino acid to be incorporated is methionine. This is true for bacteria as well as humans. Thus without the ability to resynthesize methionine, we would have to obtain much more of it in our diet; protein synthesis could be severely compromised. Insufficient amounts of B12/Co2+ in the diet can lead to a potentially fatal condition known as pernicious anemia. Not only could we not resynthesize methionine without cobalt, but we also could not completely metabolize certain fatty acids we get when we eat plants.
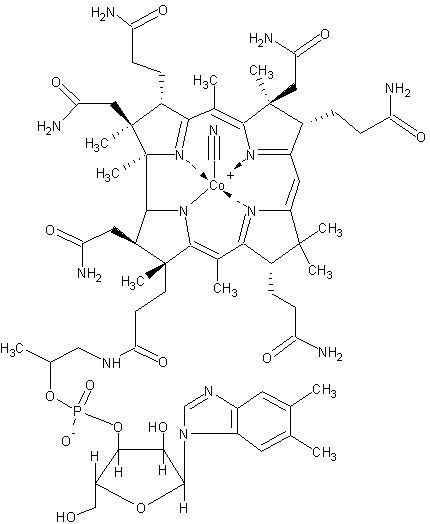
Figure 3 shows part of the vitamin B12 containing Co2+. Image by MarkusZi, via Wikimedia Commons.
Copper (Cu2+/3+)
Copper, another transition metal, is best suited for use by superoxide dismutase, an enzyme critical for the destruction of toxic oxygen free radicals. An iron-copper center also serves as the final electron donor to oxygen in the cytochrome oxidase complex, the final component of the electron transport chain in mitochondria. The process of photosynthesis also requires copper to be complexed to various proteins serving as electron carriers.
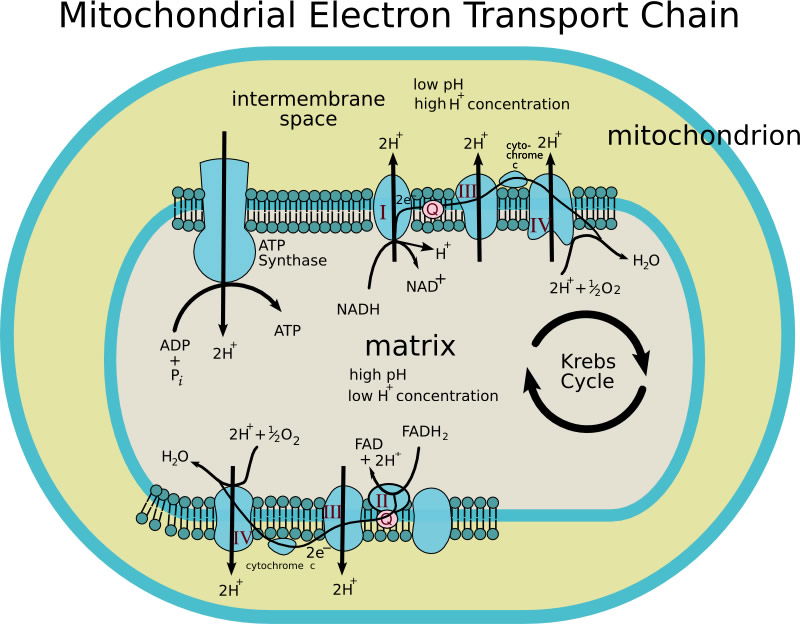
Figure 4 shows a mitochondrion and the electron transport chain along the inner membrane. Image by Patho, via Wikimedia Commons.
Manganese (Mn2+)
Manganese is not as commonly used as some of the other transition metals, but where it is used is of extreme importance. The enzyme superoxide dismutase has a mitochondrial form of the enzyme, which uses Mn2+ rather than a copper-zinc complex used by the cytoplasmic form.
Manganese, along with calcium, plays an integral role in the photolysis of water molecules by photosystem II in plants undergoing the “light” reactions of photosynthesis. This set of reactions long baffled scientists because they knew it takes a lot of energy to photolyze water into oxygen and hydrogen. Yet God, in his infinite wisdom, devised a mechanism using a specific arrangement of Ca2+ and Mn2+ atoms and the energy from sunlight.
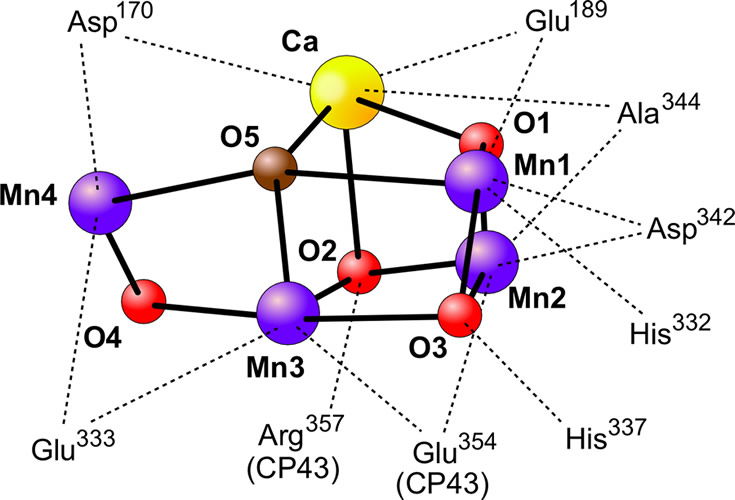
Figure 5 shows the Ca2+-Mn3+/4+ center of photosystem II which is responsible for the photolysis of water into H+s, electrons, and O2. Image by Yikrazuul, via Wikimedia Commons.
Alkali and Alkaline Earth Metals
These metals are found in groups (columns) I and II, respectively, of the Periodic Table. Sodium (Na+) and potassium (K+) have been designed with particular membrane permeabilities, allowing them to be utilized in neuronal conduction and, thus, the transfer of information throughout a body.
Dissolved small ions, such as the nonmetal, chloride (Cl-), and the metals Na+ and K+, play an important role in forming counterion “clouds” around large macroions such as proteins. These counterions help to regulate the strength and type of interactions between macroions. If the concentration of these small ions is too low, macroions may engage in repulsive or attractive interactions that could be injurious to the cell. If the concentration of the small ions is too great, required interactions may not occur. Thus the concentration of these ions must be such as to allow not only the necessary interactions between macroions, but also interactions with the proper strength. The Creator has designed each cell type to maintain just the right concentration of small ions to permit the proper functioning of all its molecular components.
The Cl- ion is not a metal, but it binds to and stabilizes the deoxy form of hemoglobin and thus helps to promote oxygen unloading to the tissues.
Magnesium (Mg2+)
Magnesium is an alkaline earth metal of group II in the periodic table. The electronic configuration of magnesium makes it uniquely suited for the process of photosynthesis whereby green plants capture and utilize the energy of the sun to synthesize the biomolecules animals use for food. No other metal has the same light-absorbing properties as magnesium; the light absorption of iron is several thousand times less. A secondary benefit of having magnesium is that magnesium also absorbs light in the near-ultraviolet/violet range. These wavelengths are strong enough to generate oxygen free radicals, which are potentially lethal to the cell.
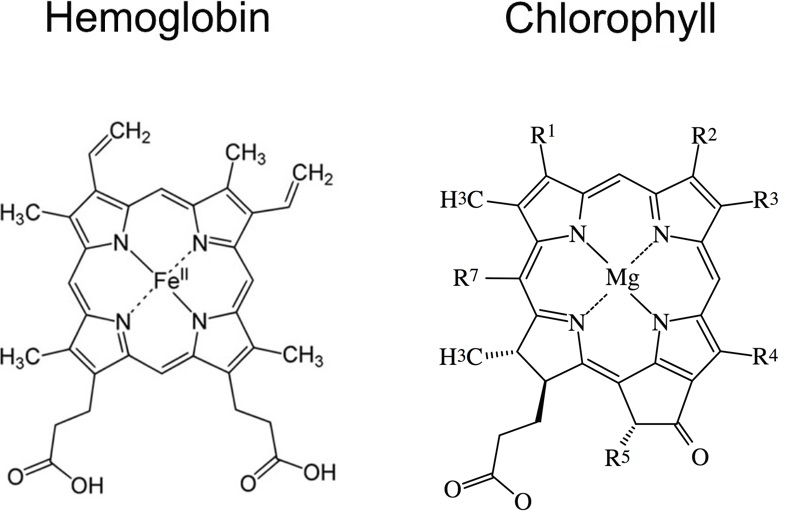
Figure 6 shows a comparison between the heme groups of chlorophyll and hemoglobin. As can be seen, they are very similar, but contain different metal atoms in their centers. The Rs show attachment sites for various groups that distinguish between one type of chlorophyll molecule and another. Not shown are the two proteins to which these prosthetic groups are attached. The proteins are very important in that they provide just the right microenvironment for these two groups function. Image adapted from Yikrazuul, via Wikimedia Commons and Itu, via Wikimedia Commons.
Magnesium is complexed to many molecules such as ATP where it aids in their stabilization. Without Mg2+ many enzymes cannot catalyze their reactions. Indeed, some regard Mg2+ as a co-substrate. Magnesium plays a vital role in the synthesis of DNA and RNA as well as proteins. It has just the right size and electronic configuration to permit the proper orientation of substrate molecules in enzyme-active sites. In some cases, cobalt can substitute for Mg2+, but then specificity for a specific substrate is compromised. If enzyme specificity is compromised, then all the reactions in the cell become chaotic. That is to say, that cells would not have the high degree of regulation as to which reactions should take place and which should not. This would result in malfunction of cellular systems and death of the cell.
Manganese (Mn2+)
Manganese is the metal of choice in that part of photosynthesis that takes water and separates the oxygen from the hydrogen, releasing the oxygen to the atmosphere for other organisms to breathe in and use for oxidation of their food.
Calcium (Ca2+)
With few exceptions, no metal plays such an integral role in so many diverse processes of a cell as calcium. Calcium is an essential structural component of bone, providing it with the rigid strength necessary for supporting body weight and for muscle attachment involved in movement and locomotion. It is the essential metal used in blood clotting, binding to and activating the various clotting factors involved in this process. All neural transmission across a synapse is dependent upon the intracellular Ca2+ concentration. Low Ca2+ would compromise neural transmission. Likewise, too much Ca2+ would also adversely affect neural transmission. Many cells use calcium as an intracellular “second messenger.” A second messenger is a small, diffusible molecule, or ion, found inside cells. Certain hormones, when bound to their target cells, will elicit the release of Ca2+ inside the cell. Calcium then binds to a protein known as calmodulin, and the Ca2+-calmodulin complex then binds to and activates various other proteins, leading to a cascade of cellular events, culminating in a response by the cell. The calcium second messenger also aids in activating a protein kinase C, which adds phosphoryl groups to other proteins, leading to either their activation or their inhibition. Calcium is absolutely essential for muscle contraction. All three types of muscle (skeletal, smooth, and cardiac) rely on proper Ca2+ concentrations in the cells. In the heart the strength of contraction is directly dependent upon the Ca2+ concentration; the higher the concentration, the stronger the contraction.
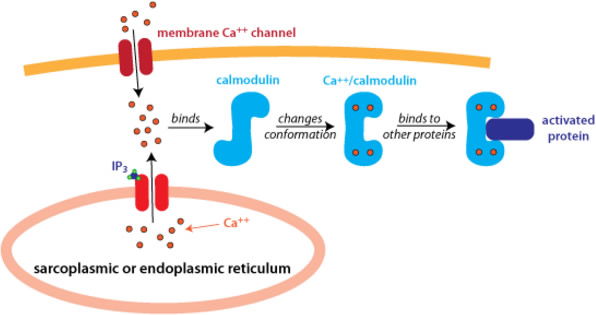
Figure 7 shows two means by which Ca2+ (red dots) can enter a cell. Calcium binds to a molecule of calmodulin (blue “S”). The Ca2+-calmodulin complex then binds to and activates a protein (purple rectangle). The activated protein can then interact with other proteins in the cell leading to a cascade of biochemical events in the cell (not shown). Image from Proteopedia.org.
In many animals when an egg is fertilized, calcium enters the egg cytosol and initiates development of the embryo.
Conclusion
Not all metals are designed to be integral parts of living organisms. Indeed, part of the habitability of earth is the fact that many of the elements have more than one purpose and are designed to be used by man to construct buildings for shelter, vehicles for transportation and countless other items which together extend habitability of various regions on earth that would otherwise not be very hospitable to life.
Much more can be said, but space will not permit. However, a study of the roles that metals play in life shows that each has been uniquely designed by a wise Creator to carry out specific and complex functions essential for life.
This completes our brief look at some of the many aspects of our planet that lead us to conclude that the earth truly was formed to be inhabited. Each of the aspects covered, it may be argued, is coincidental, but when taken together, the “coincidences” all point in the same direction—purposeful design. The arguments against a creator become severely weakened. To deny this is to be willfully ignorant of the many “fingerprints” God has left for us to find throughout his creation.
Acknowledgements
I wish to thank Ms. Sabrina Hebron for her many helpful suggestions.
References
Costanzo, Linda S. Physiology, 6th edition. Philadelphia, PA: Elsevier, 2018.
Denton, Michael. Nature’s Destiny. New York, NY: The Free Press, 1998.
Garrett, R. and C.M. Grisham. Biochemistry, 6th edition. Boston, MA: Cengage Learning, 2017.
Henderson, Lawrence J. The Fitness of the Environment. New York, NY: The MacMillan Company, 1913.
Footnotes
- Michael Denton, Nature’s Destiny (New York, NY: The Free Press, 1998), 199.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2024 Answers in Genesis