Does New Mouse Experiment Explain How Humans Lost Their Tails?
The Tail of Mice and Men
In the ongoing search for evidence of evolution, researchers seek to determine how and when humans lost their tails. After all, Old World monkeys and New World monkeys have tails, while apes and humans—known collectively in secular terms as hominoids—do not. According to the premise of evolution, there should be an ancestor somewhere back there that lost its tail on the way to becoming bipedal. Perhaps a mutation? Perhaps some unique genetic element that would differentiate between tailed monkeys and tailless hominoids? And, indeed, a group of researchers at NYU Langone Health recently published a paper, albeit a preprint that has not yet undergone peer review, titled “The genetic basis of tail-loss evolution in humans and apes.”1
While the title claims to have solved the puzzle, a deeper look reveals they may have found, at best, a piece of the puzzle. It’s like one of those hundreds of pieces of blue sky in a puzzle. The piece fits, but it doesn’t give you the whole picture. This study identified a unique genetic difference between tailed and tailless primates. When they induced these changes in a mouse model—or at least what they believed to be the genetic consequence of these changes—they produced mice that didn’t produce tails . . . or didn’t produce complete tails . . . or at least not all the time. Interesting, right? And so, I began to investigate their findings in earnest. It would be a deep dive into genomics as well as embryology. I would learn a lot about the tail of mice and men.
“Junk” DNA: One Man’s Trash Is Another Man’s Treasure
One of the interesting things about this study is where they located the possible puzzle piece. They looked where other mainstream, secular scientists—because of faulty evolutionary ideology—haven’t traditionally bothered to look. They rummaged through the “trash” of the genome. Let me explain.
It would appear that what man has considered junk for some time is turning out to be quite a treasure!
For a long time now, we have understood that all the unique instructions for any given organism are found in its DNA. From Gregor Mendel and his pea plants to the groundbreaking discoveries of Watson and Crick, we have been unraveling the mystery of DNA since the mid-nineteenth century. For decades, we have realized that only portions of our DNA code for proteins. In the 1970s, the non-coding region was dubbed “junk” DNA.2 With an evolutionary mindset, it was thought that all this “junk” was the remnant of billions of years of genetic tinkering. But since the completion of the Human Genome Project (HGP) in 2003 and then the Encyclopedia of DNA Elements (ENCODE) in 2012, we have come to realize just how little we actually know about genetics. As it turns out, only 1.5% of the human genome codes for proteins. Yet, advances in molecular biology in recent years have opened our eyes to a whole new way of thinking about the remaining 98.5% of the human genome. With every new discovery, we are coming to understand that there is meaning and purpose in virtually every base pair. Within this vast, largely ignored realm of our DNA lies complex regulatory mechanisms that direct the synthesis of proteins in the coding regions. As we unravel one layer of complexity, we discover another. It would appear that what man has considered junk for some time is turning out to be quite a treasure!
Detecting Differences in DNA
New, more advanced DNA-sequencing technology is allowing us to quickly add to the library of complete genomes for individual organisms. Comparative genomics, then, allows us to compare what is similar and what is different between different species, different genera, different families, etc. Evolutionists contend that this will be the key that unlocks the path of evolution over the millennia. So, that is precisely the tool these researchers chose to use as they pursued the case of the missing tail. They set out to try to identify a gene(s) that differed between tailed monkeys and tailless hominoids.
As they lined up the genomes for various primates, the first place they looked for differences was where everyone would expect the difference to be—in the 1.5% that codes for proteins. Yet, after comparing the 31 genes thought to be involved in tail formation (turns out the genetic contribution to heritable traits is far more complex than old Gregor Mendel ever thought), they came up empty-handed. They then turned their sights to the mysterious 98.5%—they began to comb through the “junk” pile. And it was there that they did discover a unique difference between the tailed and the un-tailed. They found a hominoid-specific element (AluY) inserted into a non-coding region of a gene associated with tail formation (TBXT).
Interestingly, there was another Alu (AluSx1) some distance from AluY that could be found in all simians—monkeys, apes, and humans. These two Alu regions together formed an inverted repeat pair, which means that they could potentially “zip” together and make a stem-loop structure that could hide a length of genetic information found in the TBXT gene (exon6 or E6) (Figure 1).
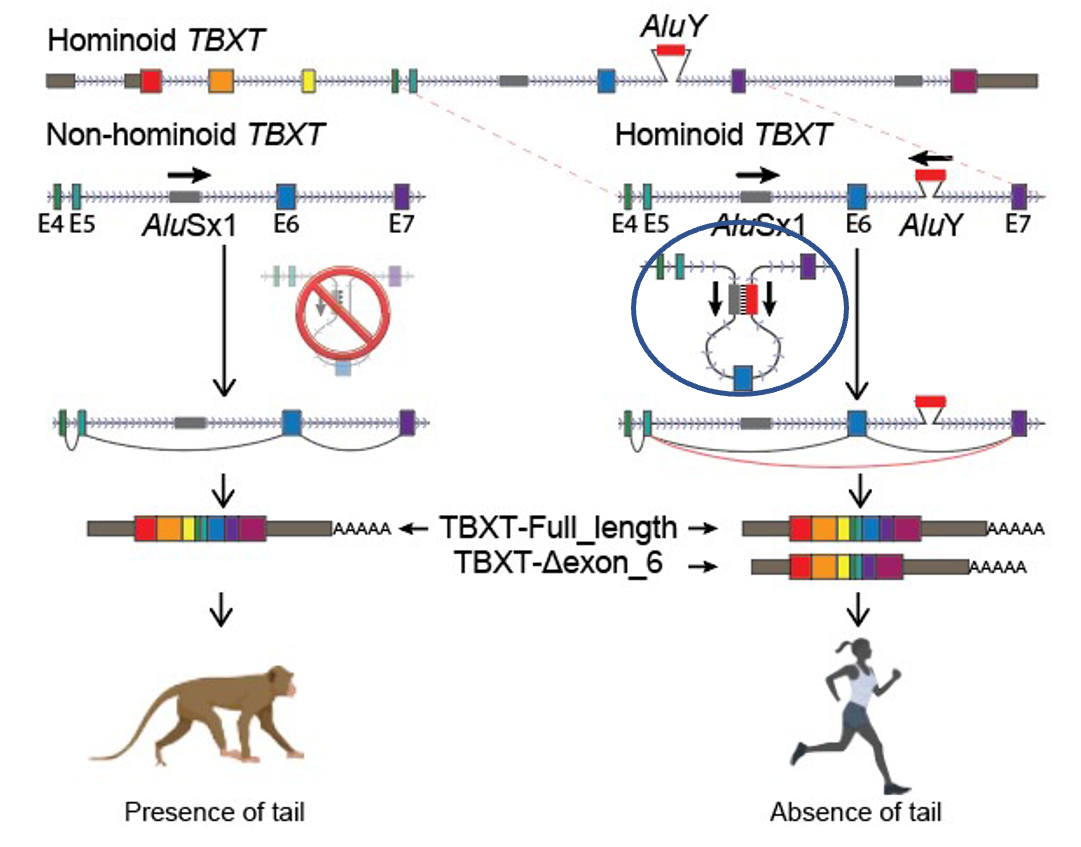
Figure 1
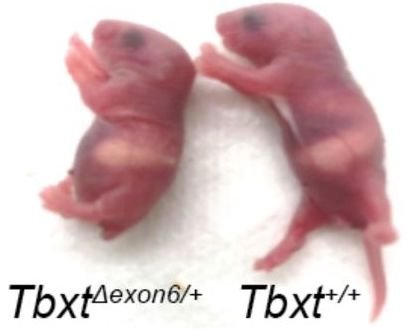
Figure 2
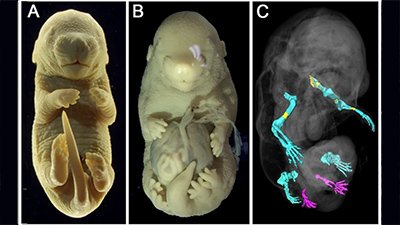
The authors of the paper surmised that if exon6 was critical for tail formation, perhaps the presence of the AluY—conjoining with AluSx1 and excluding that critical information—is what led to hominoids “losing their tails.” To test that hypothesis, these researchers did experiments in a mouse model where they removed exon6 from the mouse genome, and lo and behold, at least some of the mice were born without tails, or with shorter or kinked tails (Figure 2). These results inspired the bold claim made in their title that they had discovered “the genetic basis of tail-loss evolution in humans and apes” (Figure 3).
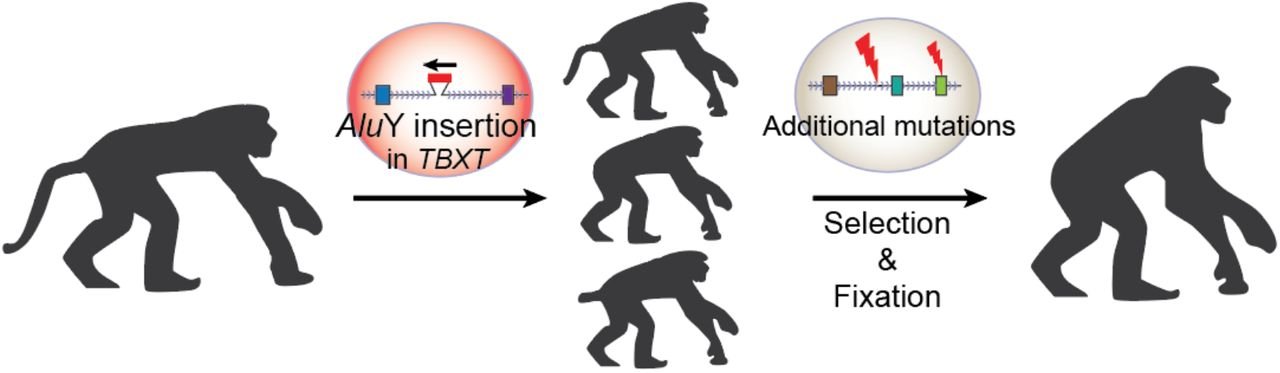
Figure 3
But Wait a Minute
Before we jump to such far-reaching conclusions, there are a number of issues to address and another lens to consider. First of all, the article fails to address where the additional genetic information comes from. They refer to the insertion of AluY into the hominoid genome, but where did it come from? We have yet to observe an evolutionary process by which we gain genetic information. There have been a number of proposed mechanisms;3 however, they are all inferred from comparative genomics with the assumption of common ancestry. They identify genetic differences between what are believed to be closely related species, and then they create hypotheses of how those changes might have come to be. For example, it is commonly believed in evolutionary circles that Alu elements are ancient retroviral insertions. These supposedly ancient invasions of the primate genome have been considered part of the genetic detritus of eons of chance mutations. It seems ironic then that what has been called “junk” for so long is now being given crucial functionality, even being touted as “the genetic basis” for tail-loss in humans. If you think about it, this “random” insertion had to just “by chance” form an inverted repeat pair with an existing Alu (AluSx1) that, “luckily,” would exclude exon6. That’s a lot of convenient happenstances. What they fail to consider is the possibility that those differences are there because, during creation week, God wrote unique instructions for each kind. Within each kind, he included a lot of genetic diversity so that organisms could survive and thrive in all the unique environments they would encounter here on earth. We are learning more all the time about how that mysterious 98.5% and other peripheral controls, known as epigenetics, can regulate genetic expression and the resulting phenotypes.4
It is also important to note that in the mouse model, they did not insert an AluY into the mouse genome, which would have more closely mimicked what is observed in the human genome. Rather, they totally removed exon6. While this may achieve their desired effect—preventing its expression—it could have broader, unanticipated effects. The more we learn of the sophisticated regulatory effects in genetics, it is altogether possible that we may find exon6 is involved in other aspects of vertebrate development as well. It is possible that this exon may be skipped at some stages of development and expressed at others. So, by eliminating exon6 altogether, it may not simulate what is actually happening in humans with both exon6 and AluY.
Furthermore, they only observed tail loss in 4 of 63 mouse pups that had been genetically altered—that’s only 6%. Some pups exhibited shorter tails (~14%), and some had kinked tails (~13%). The remaining 67% all had normal mouse tails. It is important to note that these results all come from heterozygous mice—mice that had one chromosome altered to exclude exon6 with the other remaining wild type. Homozygous mice that had both chromosomes altered all died prior to or at birth due to significant spinal cord malformations. Based on these observations, the authors suggest that the selection for the loss of a tail in humans came with an adaptive cost, that of potential neural tube defects such as spina bifida. Yet spina bifida occurs in less than 0.04% of live births.5 According to these researchers’ findings in mice, we should see far more incidence of spinal cord malformations . . . and tails!
Figure 4
The authors’ conclusions seem to infer that, as this suppposed Alu insertion entered some ancestral strain of hominoid, they would have had to be heterozygous for TBXT (Tt) in order to survive and exhibit the tailless state. If this is true, there would have been a broad range of phenotypes in those initial offspring. A Punnett square (Figure 4) would suggest that one-fourth of offspring would be homozygous exon-skipped TBXT (tt)—both chromosomes would have AluY, theoretically skipping over exon6—meaning one-fourth would not survive. One-half of offspring would be heterozygous (Tt)—expressing both full-length and exon-skipped isoforms—but in the mouse model only 6% of these were actually tailless. The remaining one-fourth of offspring would be homozygous full-length TBXT (TT)—not having the inserted AluY—and have a tail. Combined, that would imply that 96% of the offspring that survived would have had some type of tail! It is difficult to envisage how that 4% could have somehow become the prevailing—indeed, exclusive—phenotype in humans.
The authors refer to some elusive evolutionary mechanism for the “selection and fixation” of this tailless phenotype (see Figure 3, above), but we have yet to elucidate just how this process might work. In another species affected by mutations of the TBXT gene, the Manx cat, we see the same type of results observed in the mutant mouse model and what would be expected based on the Punnett square illustration used above. The pairing of two Manx cats results in lower viability rates in offspring and a dramatic increase in altered tail phenotypes, as well as various neural tube defects, urinary and fecal incontinence, and locomotor disturbances of the pelvic limbs.6 We don’t see any evidence of fixation, and it is unlikely that the Manx cat is on its way to becoming bipedal! Proponents of evolution would suggest that a few more million years might allow for the random arrival of other mutations that will somehow result in the brilliant functionality that we observe in the genetic code today. This seems rather a convenient distraction from the actual genetic consequences of the supposed mutations during that million-year waiting period: deformity and death.
Some may suggest that we still have throwback events where humans present with a tail. And yet what some try to call human “tails” are extremely rare—usually a bulging or protrusion associated with spinal malformations referred to as “pseudotails.” What they call “true tails” are even more rare and have had to be redefined in a way that doesn’t require vertebral involvement. A “true human tail,” according to the current definition, “contains adipose tissue, connective tissue, central bundles of striated muscle, blood vessels and nerves and is covered by skin. Bone, cartilage, notochord and spinal cord are lacking. . . . Unlike the tail of other vertebrates, human tails do not contain vertebral structures.”7 It’s interesting that they had to come up with a new definition of tail in order to try to demonstrate that humans can exhibit what they refer to as “vestigial” tails.
In summary, if this supposed AluY insertion is “the genetic basis of tail-loss evolution in humans and apes,” it’s interesting that we don’t see far more miscarriages and neural tube defects—and far more tails! Long, short, kinked, or otherwise. A far more likely scenario is that the AluY is strategically positioned in both humans and apes—regulated by complex genetic controls—to provide the structure and function most suited for these unique creations and their God-intended habitats and lifestyles. It is quite possible that as we learn more of the regulatory aspects of Alu elements in the human genome, there may be selective expression of exon6 at various stages of embryonic development.
I have heard it said that sometimes the best thing we can do as creationists is to simply wait—to allow time for the data to clarify itself as we continue to unveil the treasure in the “trash.” Because truthfully, all we really know is that this section of DNA is somehow involved in the formation of a tail . . . in mice . . . and Manx . . . and other tailed vertebrates. To assume that the expression of exon6 in humans would result in a tail is purely speculative and quite a leap. After all, just how and when does a tail form anyway?
Frame and Form
It’s always good to start at the foundation, so let’s begin by taking a look at the general development process itself—specifically in amniote vertebrates (reptiles, birds, and mammals). Upon fertilization—the union of sperm and egg—a new life is formed. Not long after that, cells begin to proliferate, and through a process called gastrulation, three distinct germ layers begin to form. There is the ectoderm (outer layer, which will produce the skin and nervous system), mesoderm (middle layer, which will develop into connective tissues, the circulatory system, muscles, and bones), and endoderm (inner layer, which will form the lungs, digestive system, and urinary system). Immediately following the process of gastrulation, the embryo undergoes neurulation. This is a folding that will form the neural groove, which then becomes the neural tube and ultimately becomes the central nervous system, which is comprised of the brain and spinal cord.
Scientists are getting a glimpse of the elaborate architectural plans, and yet they continue to fail to recognize the Great Architect.
All of this early development arises from a smear of cells called the primitive streak. It’s pretty impressive if you really think about it. If we were ever to observe a pile of bricks multiply and assemble itself into a cathedral, it might be easier to believe this process happens by chance. But we know that there are great minds and detailed architectural plans that lie behind an exquisite cathedral. Scientists are getting a glimpse of the elaborate architectural plans, and yet they continue to fail to recognize the Great Architect.
Figure 5
If you are wondering why we started with developmental biology, bear with me. We are on our way to understanding the amazing process that underlies the formation of a vertebrate tail—or not. You see, forming simultaneously with the neural tube is the presomitic mesoderm (PSM). The PSM is critically involved in the formation of somites, which are literally the building blocks of the vertebrate body plan. Sequentially, in a rhythmic fashion, pairs of somites will form on either side of the neural tube. A time-lapse video shows this process literally happening right before your eyes (Figure 5).8 Like an organic zipper, the somites almost magically appear in the wake of a milieu of various transcription factors and growth factors that go before them and lay out the basic plan. These masses of mesoderm will ultimately give rise to the vertebral column, ribs, skeletal muscles, and subcutaneous tissues.
Even though many vertebrates look very similar at this stage, there are unique blueprints nested within each species—indeed, each individual—that will determine precisely what that embryo is destined to become and just how it will look. Just as any great architect will start with some pretty standard frame and form (foundations, support beams, rafters), the Great Architect has done the very same. As the building progresses, the overall design and the smallest of details begin to emerge. In both the cathedral and the camel, the blueprints dictate the design: the cathedral may have one dome or two; the camel may have one hump or two. In the case of the tail of mice and men, there may be a tail or no tail. It’s all in the plans.
Building Block by Block
With the roadmap of the somites laid out, the embryo will continue to develop. As is apparent very early on, vertebrates are segmented. There is a distinct partitioning of the body axis into a series of repeating units or segments. Molecular biology has discovered that each segment, or series of segments, is under the control of specific genes known as homeobox or Hox genes.9 These genes are encoded to produce transcription factors that will regulate how and when various proteins are made, thereby dictating the entire developmental process along the head-tail axis. Let’s consider these the general contractors for each division on a build site.
Figure 6 shows how the various Hox genes are organized sequentially along the organism’s chromosomes. In the developing embryo, each Hox gene is responsible for the development within each corresponding segment(s) —conveniently color coded in this example. For example, the Hox gene responsible for the head region will direct the synthesis of lips, beaks, or baleen. In the limb regions, the appropriate Hox gene will dictate legs, wings, or flippers. Indeed, there appears to be certain “programs” that produce these structures (e.g., a “lip” program or a “leg” program). The “vertebra” and “rib” programs are run quite a few times in the giant python—600 vertebrae means 1,200 ribs! But this is no mistake or glitch in the program. God wrote the instructions that would create the python just as he intended.
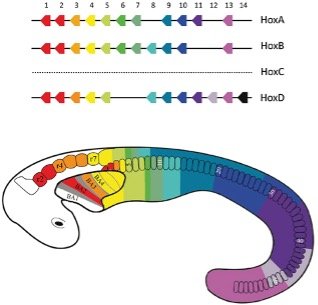
Figure 6
Paired box or Pax genes are a family of genes coding for tissue-specific transcription factors.10 These could be like the subcontractors, constructing the specific features at each build site. For example, a mouse Pax gene for the development of the eye (Pax6) inserted into the fruit fly genome develops a compound fly eye instead! Furthermore, ectopic, or “out of place,” eyes have been stimulated to develop in the fruit fly antennae, wing, and leg. (Yes, scientists have actually done this!)11 That means that the mouse Pax6 gene is similar enough to the fruit fly homolog that it still knows to make an eye, but the genetic machinery available to it in the fruit fly will make a compound fly eye rather than a mouse eye. And it will make it wherever it is “told” to! It’s like a subcontractor who may build a simple suspension bridge of logs and jute rope in the Amazon jungle and a massive bridge out of concrete girders and steel cables in Manhattan, New York. The subcontractor, skilled in building bridges, uses the plans and the materials available at the specific worksite. In the case of this biological “construction,” the subcontractor (Pax6) showed up on a fruit fly construction site. It built an eye based on the plans found at the worksite, with the materials found there. Simply amazing!
These general contractors (Hox genes) and the subcontractors they oversee (Pax genes) are, ultimately, responsible for the development of the tissues and organs in their segment(s)—and they get right to it. From the moment somites form, they start to mature and differentiate. They split into three major regions: the dermatome, the myotome, and the sclerotome. The dermatome and the myotome give rise to skin and muscle, respectively, while the sclerotome gives rise to the vertebrae of the vertebral column and ribs. As we follow the tail of mice and men, we must pursue the fate of the sclerotome.
The Tale of the Human Tail
So, as we build our organism block by block from lips to legs, we’re getting closer to tails. Tails are typically defined as an extension of the vertebrae—any number of extra caudal vertebrae, generally extending beyond the rest of the body. This can range from as few as 3 (in the human “tailbone” or coccyx, which technically doesn’t even extend beyond the rest of the body) all the way up to 47 (in the aptly named long-tailed pangolin). As the somites are sequentially produced, there are a number of factors that will dictate how many will be made. Molecular biologists have identified various growth factors and suppressors that control the termination of axis elongation.12 There is a reduction in the size of the PSM as well as many other signals that determine precisely when to stop making somites. This, ultimately, determines how many vertebrae will form—including the caudal vertebrae comprising any type of tail—and the process is uniquely regulated by this yet-to-be-discovered Master plan for each organism.
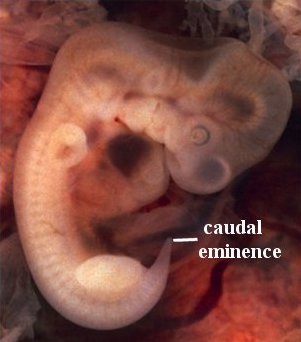
Figure 7
For humans, the tale of the “tail” unfolds between weeks five and eight of development. At week five, a structure appears in all vertebrates that is referred to as the tail bud. It has little to do with an actual tail, but rather is a mass of undifferentiated (pluripotent) cells at the posterior end of the embryo. It is more appropriately referred to, in humans, as the caudal eminence (Figure 7). This mass of undifferentiated cells, transcription factors, and growth factors precedes the organized somites and the earliest aspects of the nervous system. By six weeks, the somites are primarily laid down, the “tail bud” begins to disappear, the sclerotome has differentiated, and a process called chondrification begins. Basically, cartilaginous templates of the vertebrae are laid down. By week seven, ossification, the process of replacing cartilage with bone, begins. By week eight, the “tail bud” disappears.
In addition to mental images conjured from the ill-named and short-lived “tail bud,” another tale of the human tail comes from the observation that during somitogenesis there is a time when there are 8–10 caudal somites.13 At birth, only the coccyx remains, which is only 3–5 (usually 4) fused caudal vertebrae. So, what happened to all the extra vertebrae? What happened to the embryonic “tail”? The fact is, there never were any extra vertebrae. There was no human tail. Those extra somites were resorbed prior to ossification. While we are unsure why those extra somites might be needed during such a narrow window of development, we can trust that there is a purpose behind their presence. The extra somites are resorbed via a process called apoptosis, or programmed cell death.14 There is a plan in place to remove those undifferentiated segmental structures once there is no longer a need for their presence—much like scaffolding may be needed during a building project but is removed once it is no longer necessary.
So, even while the TBXT gene is highly conserved in vertebrates—with human and mouse protein sequences sharing 91% identity with a similar exon/intron architecture,15 the entirety of the complex machinery in the human embryo is set up to build a human. Just as Pax6 is the subcontractor involved in the building of eyes, TBXT appears to be a subcontractor in the building of tails, at least in organisms that have one. This is an important distinction. Because, more precisely, TBXT is involved in the building of the posterior segments of an organism. In humans, our instructions call for a fused sacrum and a coccyx. And there is purpose and function in that structure—serving as an anchor point for important muscles that form the pelvic diaphragm. This bowl-shaped muscular floor keeps our internal organs from literally falling out and plays an important role in defecation as well.16 So, even if we were to somehow unveil and transcribe the information found in exon6 that may be excluded by AluY, it is quite possible that it would result in changes to the posterior axial development—possibly extra somites, possibly extra vertebrae—but that does not necessarily mean it would produce a tail. It is perhaps more likely that there would be spinal deformations capped by a human “tailbone” or coccyx.
Parting Thoughts: Brilliant Work . . . Questionable Conclusions
Through my work here at Answers in Genesis, I am often asked to review current research articles that make extraordinary claims that supposedly support evolution. In doing so, I have the opportunity to conduct an in-depth investigation of the literature and read a lot of the latest research going on in various areas. This particular article required a deep dive into the realm of developmental biology, comparative genomics, and genetic regulation. As I pored over the vast amount of knowledge we have accumulated in these areas as they relate to the ultimate design of each organism—including if and what kind of tail they may end up with—I didn’t find myself being convinced of evolution. Rather, I found myself in awe . . . again.
I stood in awe of God as a brilliant Creator, and I stood in awe of the pinnacle of his creation: mankind. Brilliant men and women, created in his image, continue to unravel the mysteries of genetics and embryology. Yet, the wealth of knowledge we have accumulated is still clearly just the tip of the iceberg. We are just beginning to delve into the treasure trove of the 98.5%—the vast majority of DNA that was, for far too long, considered simply to be “junk.” The wealth of information being discovered in that 98.5% continues to unveil an intricacy and complexity that defies random chance.
From somite to sclerotome to specialized spines—unique to each created kind—it becomes clear that this whole process is far too sophisticated for random chance.
While I tip my hat to the fine work of those who have diligently teased out some of the minute details of genetic regulation of vertebrate development, I caution once again that so much of this work (pretty much all of it, really) is being done with an evolutionary worldview. Brilliant men and women who have their evolutionary glasses strapped on so tightly that they miss the mind-boggling grandiosity of the system they are slowly unveiling. Their evolutionary assumptions continue to influence their understanding and dominate the dialogue around their discoveries. There is an institutional bias that fails to consider the possibility of an almighty Creator, which leaves them trying to explain how these highly programmed, systematized, finely tuned events somehow “just happened randomly,” given enough time.
Yet, the molecular biology research I combed through from the past few decades reveals a level of complexity and cellular sophistication that is simply incompatible with the idea that random mutations could serve as the engine for evolution . . . or that evolution is even a possibility at all. Rather, as Romans 1:20 states, we can clearly see the handiwork of the Creator, God, in what has been made. From somite to sclerotome to specialized spines—unique to each created kind—it becomes clear that this whole process is far too sophisticated for random chance. If we are intellectually honest, there’s no denying it. We are without excuse. Praise be to God! May he raise up a generation of young scientists who have the minds to contribute to this fascinating field of research and the eyes to see his fingerprints all over it. There are so many more pieces of blue sky to fit into the puzzle. Eventually, the whole picture will emerge. We can work diligently in the confidence that, when it does, it will be signed in the bottom corner: With love, God.
For since the creation of the world God’s invisible qualities—his eternal power and divine nature—have been clearly seen, being understood from what has been made, so that people are without excuse. (Romans 1:20 NIV)
Footnotes
- Bo Xia et al., “The Genetic Basis of Tail-Loss Evolution in Humans and Apes,” bioRxiv (September 2021): 1–38, https://doi.org/10.1101/2021.09.14.460388.
- Nelson J. R. Fagundes et al., “What We Talk About When We Talk About ‘Junk DNA,’” Genome Biology and Evolution 14, no. 5 (May 2022): evac055, https://doi.org/10.1093/gbe/evac055.
- C. Chandrasekaran and E. Betrán, “Origins of New Genes and Pseudogenes,” Nature Education 1, no. 1 (2008): 181.
- K. Kloster, “How Grandma’s Recipes Can Remind Us of God’s Recipes for Life–Genomes,” Answers in Depth 18 (May 15, 2023), https://answersingenesis.org/genetics/how-grandmas-recipes-can-remind-us-of-gods-recipes-life-genomes/.
- Cara T. Mai et al., “National Population-Based Estimates for Major Birth Defects, 2010–2014,” Birth Defects Research 111, no. 18 (November 2019): 1420–1435.
- M. E. Deforest and P. K. Basrur, “Malformations and the Manx Syndrome in Cats,” Canadian Veterinarian Journal 20 (November 1979): 304–314.
- B. Mukhopadhyay et al., “Spectrum of Human Tails: A Report of Six Cases,” Journal of the Indian Association of Pediatric Surgery 17, no. 1 (January 2012): 23–25, https://doi.org/10.4103/0971-9261.91082; PMID: 22279360; PMCID: PMC3263034.
- N. Denans, T. Limura, and O. Pourquié, “Hox Genes Control Vertebrate Body Elongation by Collinear Wnt Repression,” Elife 4 (February 2015): e04379, https://doi.org/10.7554/eLife.04379; PMID: 25719209; PMCID: PMC4384752.
- Edward B. Lewis, “A Gene Complex Controlling Segmentation in Drosophila,” Nature 276, no. 5688 (1978): 565–570, https://doi.org/10.1038/276565a0.
- E. Dahl, H. Koseki, and R. Balling, “Pax Genes and Organogenesis,” Bioessays 19, no. 9 (September 1997): 755–765, https://doi.org/10.1002/bies.950190905; PMID: 9297966.
- George Halder, Patrick Calaerts, and Walter J. Gehring, “Induction of Ectopic Eyes by Targeted Expression of the eyeless gene in Drosophila,” Science 267 (March 1995): 1788–1792, https://doi.org/10.1126/science.7892602.
- O. Pourquié, “Vertebrate Segmentation: From Cyclic Gene Networks to Scoliosis,” Cell 145, no. 5 (May 2011): 650–663, https://doi.org/10.1016/j.cell.2011.05.011.
- “Embryology and Development,” Radiology Key, accessed May 19, 2023, https://radiologykey.com/embryology-and-development/#bib1.
- A. K. Voss and A. Strasser, “The Essentials of Developmental Apoptosis [version 1; peer review: 3 approved],” F1000Research 9 (February 2020): F1000 Faculty Rev: 148, https://doi.org/10.12688/f1000research.21571.1; PMID: 32148779; PMCID: PMC7047912.
- Y. H. Edwards et al., “The Human Homolog T of the Mouse T(Brachyury) Gene; Gene Structure, cDNA Sequence, and Assignment to Chromosome 6q27,” Genome Research 6 (1996): 226–233.
- “Coccyx,” Radiopaedia, accessed May 18, 2023, https://radiopaedia.org/articles/coccyx.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2024 Answers in Genesis