Endogenous Retroviruses: Key to Mammalian Brain Development?
News to Know
News Sources:
- ScienceDaily, “Do viruses make us smarter?”
- Scientific American, “Ancient Viruses Gain New Functions in the Brain”
Did viral invasion help the mammalian brain evolve?
About 8–10% of human DNA, as well as the DNA of animals like mice, consists of scattered DNA sequences matching those of retroviruses. These sequences are called endogenous retroviruses (ERVs) because they are actually a part of the healthy host cell’s DNA. (Exogenous retroviruses—like HIV that causes AIDS—come from outside a cell and infect it.) How ERVs came to be part of our DNA and what they are doing there have been the subjects of much speculation and research. Scientists have known for some time that placental formation depends on ERVs. Evolutionists credit such viruses with making mammalian evolution possible. Now scientists have shown that ERVs play a crucial role in the development of the mouse brain.
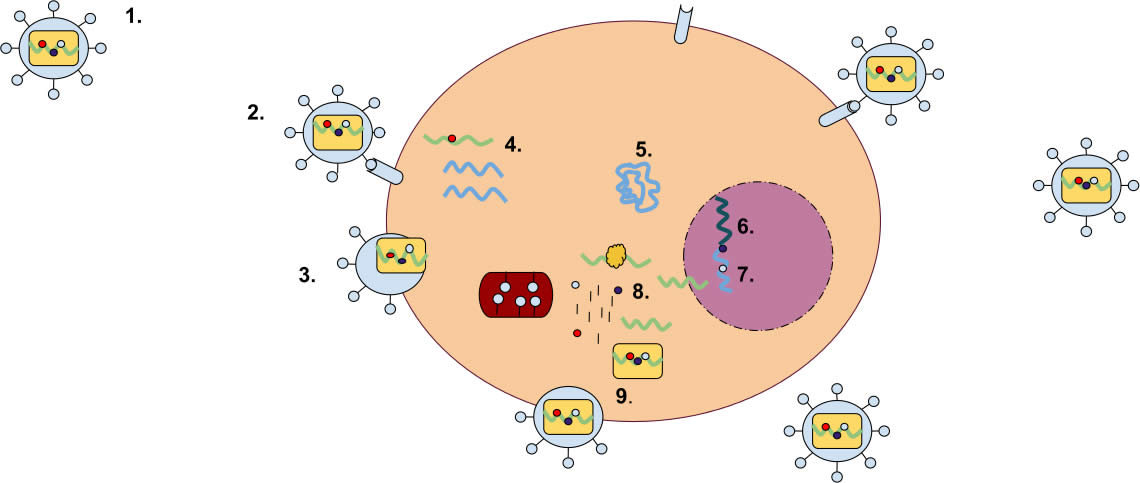
Exogenous retroviruses invade cells. They make DNA copies of their own RNA genome, and each DNA copy inserts itself into the host cell’s genome, where it is called a provirus. Once incorporated into a cell’s DNA, this provirus may replicate itself and move on to infect other cells or it may linger for a time. Proviruses can also be inherited as a part of a host cell’s genome; an inherited provirus is called an endogenous retrovirus. A provirus—whether exogenous or endogenous—can affect the expression of nearby genes. And if a virus is incorporated into the genome of a reproductive cell, it can be passed down to offspring. Evolutionists think that endogenous retroviruses are broken-down versions of ancient exogenous retroviruses that were incorporated into many genomes millions of years ago. Reproduced from user “Mrdavis21,” Wikimedia Commons.
What’s a Virus to Do?
“We have been able to observe that these viruses are activated specifically in the brain cells and have an important regulatory role,” explains Johan Jakobsson, who led the Lund University team that made the discovery. “We believe that the role of retroviruses can contribute to explaining why brain cells in particular are so dynamic and multifaceted in their function. It may also be the case that the viruses’ more or less complex functions in various species can help us to understand why we are so different.”1
Many viruses contain DNA, but retroviruses contain RNA instead. An RNA virus normally makes DNA copies of its genome and inserts them into a host cell’s DNA. Exogenous retroviruses are RNA viruses that invade cells and, like DNA viruses, hijack the host cells’ machinery to replicate themselves and then go on to infect other cells. But endogenous retroviruses (ERVs) do not behave like this. They—or rather the DNA versions called proviruses—are actually part of the cells’ genome. They do not seem to be in the business of copying themselves and breaking free to infect other cells but simply get copied along with the rest of the DNA and passed along from generation to generation.2 Whether endogenous or exogenous, this retroviral DNA can trigger the expression of other genes in the DNA. Exogenous retroviruses that do this can, for instance, cause tumor production. But what sorts of things might endogenous retroviruses do?
The Placental Story
Before exploring what Jakobsson and colleagues discovered about the brain cells in mouse embryos, let’s step back a moment and examine the role of ERVs in the formation of the placenta.
Cell membranes normally keep their cell “insides” in and the outside out. They regulate what part of the outside is allowed to come in and what part of the inside is allowed to exit. But placental formation requires cell fusion! So what did God design in order to make this happen? Something we know as a virus. Specifically, an endogenous retrovirus. Except that this virus is not an enemy alien, a pathogenic invader come to destroy, but an integral part of the mammalian genome and essential to mammalian life.
Disease-causing viruses, a scourge to mankind, insert their genetic material into host cells and hijack the cell’s reproductive machinery to replicate the components to build more viruses. Retroviruses must bring along the molecular tools to make DNA copies of their own RNA. Therefore, they must fuse with the cell membrane so that all of their components enter the host cell. Retroviruses are able to do this because they produce a particular protein product that tags the cell membrane, acting like an entry-pass by making the cell recognize the retrovirus as “friendly” and respond to it by merging its membrane with the virus’s. Endogenous retroviruses associated with placental formation also make this substance.
As a mammalian embryo forms, some of its cells are partitioned off to form the placenta. Those cells—called trophoblasts—invade the uterine wall and fuse together to form the placenta, a complex organ that builds blood vessels continuous with those of the developing embryo, connecting via the umbilical cord. In recent years scientists have learned that the ability of the trophoblastic cells to fuse, forming the placenta, comes from certain retroviral genes in the mammalian genome. The specific genes differ from species to species, but expression of these genes is essential to placental formation and therefore to mammalian reproduction.
Of course these particular retroviruses are actually part of the genome. How did mammals get these viruses? Were these endogenous retroviruses ever really invaders?
Ancient Invaders Bearing Evolutionary Gifts?
Did ERVs start out as invaders bestowing the information life needed to evolve into complex organisms like mammals? Evolutionists believe so. Evolutionists maintain that long ago retroviruses infected cells of evolving organisms, supplied those cells with the ability to fuse or do other things useful for survival or reproduction, and—like the formerly outdoor feline that now lives exclusively inside my house—stayed on like freeloaders. And unlike the cat, these ancient retroviruses provided some useful services in their new homes. Evolutionists believe endogenous retroviruses no longer break loose to infect other cells because millions of years of mutations damaged them.
Many creation scientists instead maintain that the same Designer, the creator God of all, used this design to make life and reproduction possible in many organisms, including human beings, from the beginning. If that is the case, then at least some of these “endogenous retroviruses”—regardless of their disconcerting, dangerous-sounding name—were never random invaders that took up residence in cells but a part of God’s design from the beginning.
Transposable Elements
Scattered throughout DNA, endogenous retroviruses (ERVs) were once thought to be part of the so-called “junk DNA” that evolutionists thought were evolutionary leftovers without any function. Of course, now scientists, with the ENCODE study, have demonstrated that much if not most of so-called “junk DNA” has a function. Like many other transposable elements (DNA sequences that are repeated in many places in the genome), ERVs can regulate the expression of other genes. ERVs are found in all vertebrate genomes. As such, the ERVs may help explain at a genetic level how species with genetic similarities differ greatly. Of course, understanding how animals differ is not the same as demonstrating how they came to differ in the unobservable past.
Controlling Genetic Expression
The expression of genes in DNA is controlled in several ways. DNA methylation—attaching “methyl” chemical groups to DNA in order to silence certain genes—is one way. Another such epigenetic (“above the level of the gene”) method of controlling genetic expression includes wrapping the genes around spool-like proteins called histones. With such controls, genes can be expressed at the time when they are needed, such as at particular phases of embryonic development, and then be silenced.
Most known ERVs seem to be controlled by DNA methylation. But, Jakobsson says, “There seems to be a different mechanism regulating endogenous retroviruses in brain cells than in other cells.”3 These cells control the expression of their ERVs with histone modification.
Architects of Neural Complexity?
Early in embryonic development, these embryonic brain cells may, researchers suspect, fine-tune the way nerve cells develop and the complex connections they form using ERVs. “Brain cells are very complex compared to other cells,” explains Jakobsson. “Co-opting endogenous retroviruses allows for much more complexity, especially since they make up so much of the genome.”4
Jakobsson’s team found that a gene called TRIM28 is responsible for the histone modification of ERVs in mice. They experimented with disabling TRIM28 genes to see what would happen. In most cells, the loss of histone modification made no difference. But in mouse brain cells the effect was dramatic. When TRIM28 is disabled in the embryonic brain cells, their ERVs seemed to “wake up” and trigger expression of various nearby genes and even the transcription of some other non-coding regions of the DNA.
When both copies (alleles) of TRIM28 are destroyed in living mouse embryos, they die. When only one copy of TRIM28 is eliminated, the embryos survive but have various behavioral abnormalities such as hyperactivity and symptoms mimicking human psychiatric disorders. This suggests, the researchers believe, that ERVs could not only be important for mammalian embryonic brain development but even be associated with behavioral problems:
Together, these findings demonstrate that disruption of TRIM28 levels in the mouse brain results in behavioral changes that are similar to impairments found in humans with certain psychiatric disorders. With this in mind, it is noteworthy that increased levels of ERV transcripts have been detected in patients with several neurological and psychiatric disorders.5
Functional Significance
Many scenarios are possible. ERVs may play a crucial role in early embryonic brain development—perhaps by helping orchestrate other complex controls on gene expression. And they might ultimately cause problems if not shut down properly once their job is done. It is far too early to draw any definitive conclusions about the functional significance of ERVs in normal and abnormal brain development and function, even in mice, much less in humans. And it will be difficult to nail down too many specifics, as the same ERVs appear at many locations in the genome, multiplying the potential complexity of their effects. Nevertheless, it now seems likely that the ERVs do play some sort of role in mouse—and possibly all mammalian—embryonic brain development.
The next question to address, however, is where these ERVs came from. And if they somehow help tweak and tailor the complex development of the embryonic brain, does that mean they played a role in the evolution of the complex mammalian brain? Evolutionists often claim they can extrapolate from observations of embryonic development to unobservable speculations about a hypothetical evolutionary past. (This is called Recapitulation Theory. Read more about in “Recapitulation Theory: Repackaged & Re-Applied.”) Indeed, since ERVs comprise a significant percentage of the mammalian genome and are necessary for proper placental development, do we, as biological mammals with superior brain function, owe our very existence to the intrusion of ancient retroviruses on the evolutionary process? Many evolutionists would answer “yes.”
Evolutionists generally assume that ERVs are the hobbled remains of retroviruses that invaded the genomes of evolving organisms millions of years ago, bringing in essential genetic information to facilitate evolution. Jakobsson and colleagues speculate about the possible evolutionary pros and cons of ancient retroviral incorporation into genomes:
Occasionally, retroviruses infect germline cells allowing the integrated proviruses to be passed on to the offspring as an endogenous retrovirus (ERV). Around 8%–10% of the human and mouse genome are composed of this type of TE [transposable element], and, despite up to millions of years since their integration in host germline, many ERVs contain sequences that can serve as transcriptional start sites or as cis-acting regulatory elements in the host genomes. The large amount of ERVs in mammalian genomes suggest that they play important roles in the host organisms, for instance, by influencing gene regulatory networks, but ERVs have also been linked to diseases. In humans, aberrant expression of ERVs has been found in both cancer and brain disorders, although causality remains to be established. Thus ERVs may contribute both beneficial and detrimental effects, which have been balanced throughout evolution, to the host organism.6
We must remember, however, that no scientist has observed these ubiquitous proviral elements being integrated into vertebrate genomes, nor can anyone show that these events occurred millions of years ago. DNA sequences do not come with labels giving their age. Efforts to say how many millions of years old a particular gene is are based on a stack of unverifiable evolutionary worldview-based assumptions.
Dr. Yingguang Liu, associate professor of microbiology at Liberty University’s College of Osteopathic Medicine, commented on the significance of this study and how it is explained by the biblical creationist model of origins:
For a number of years, I have been advocating that ERV elements were created in the cell to provide coordinated regulation of interspersed genes, among other possible functions. Our laboratory studies showed that multiple human ERV elements were controlled simultaneously by female sex hormones. It is encouraging that mouse ERVs are found to regulate nearby genes in the brain. However, these authors still believe ERVs are recent insertions into the mouse genome. Did mice all have psychiatric/behavioral disorders before they were infected by ERVs?7
Many ERVs are not only critical to life through irreducibly complex associations with other cellular processes but are also tightly controlled by the molecular mechanisms that normally control genetic expression in cells. This suggests they were a critical design element present from the beginning—not of molecules-to-mammals evolution, but of divinely designed creation.
Viruses in a Very Good World?
So if these important ERVs are not the vestiges of viral invasion representing a pivotal event in evolutionary history, why do they look like viral elements? Where did viruses come from anyway? Do they serve any good purposes?
Well, as much as we are accustomed to thinking of viruses as our enemies—especially in the flu season and more particularly in view of the terrible scourges of viruses like HIV and Ebola—we need to remember that many viruses do not hurt us. In fact, many viruses serve useful roles. For instance, bacteriophages—viruses that infect bacteria—help our immune system fend off pathogenic viruses. Bacteriophages may also facilitate the transfer of genetic information between microorganisms, enabling them to adapt to a changing environment. And since most microbial organisms are actually beneficial and even essential to us and to our environment rather than harmful, the fact that God apparently designed microbes to adapt in this way is an example of God’s good design. That some viruses, bacteria, and other microbial organisms have become pathogenic is an effect of sin’s curse on a world originally created perfectly good (Genesis 1:31).
Much research remains to be done to discover the functions of ERVs and to learn more about how today’s pathogenic viruses could be related to non-pathogenic counterparts. Some scientists—both evolutionists8 and creationists—have even suggested that endogenous retroviruses could be the source of some exogenous retroviruses! But we do know from God’s eyewitness account in the Bible that God created all kinds of living things to reproduce after their kinds about 6,000 years ago. Even though viruses were first discovered in connection with disease, we should not discount their importance in a perfectly good world. And given that the same Creator—our common Designer—created mammalian genomes and viruses, the existence of homologous DNA sequences among them should not be a surprise. Perhaps as more research is done, clues about possible relationships between endogenous and exogenous retroviruses will become apparent.
For more information:
- Were Retroviruses Created Good?
- Human Endogenous Retroviruses (HERVs)—Evolutionary “Junk” or God’s Tools?
- Placental Development Triggered by Genomic Parasites
- Virus “Evolution” Benefits Mankind?
- Why Did God Make Viruses?
- Mutational Secrets of a Virus
- Friendly Viruses
- “Partners in Slime”: Mucus and Our Viral Friends
- The Natural History of Retroviruses
- Is the Ebola Epidemic Evolution in Action?
- The Wonderful World of Bacteria in Your Body
Footnotes
- Lund University, “Do Viruses Make Us Smarter?” ScienceDaily, January 12, 2015, http://www.sciencedaily.com/releases/2015/01/150112093129.htm.
- In some disease states this does happen. A leukemia-causing mouse ERV, for instance, behaves this way in the laboratory, LINK https://answersingenesis.org/biology/microbiology/were-retroviruses-created-good/ but this does not seem to the norm for ERVs in healthy hosts.
- Andrea Alfano, “Ancient Viruses Gain New Functions in the Brain,” Scientific American, January 19, 2015, http://www.scientificamerican.com/article/ancient-viruses-gain-new-functions-in-the-brain/.
- Ibid.
- L. Fasching et al., “TRIM28 Represses Transcription of Endogenous Retroviruses in Neural Progenitor Cells,” Cell Reports 10 (January 6, 2015): 20–28, http://dx.doi.org/10.1016/j.celrep.2014.12.004.
- Ibid (emphasis added).
- Dr. Yingguang Liu, email message to Dr. Georgia Purdom, January 13, 2015.
- This is not idle speculation but is supported by clues in the DNA of the ERVs. Did God create exogenous retroviruses and endogenous retroviruses to work together? Are the pathogenic ones examples of good ones corrupted through mutation and other damaging events in a sin-cursed world? This is a very real possibility. See “The Natural History of Retroviruses” and “Retroviruses from Retrotransposons” to learn more.
Recommended Resources

Answers in Genesis is an apologetics ministry, dedicated to helping Christians defend their faith and proclaim the good news of Jesus Christ.
- Customer Service 800.778.3390
- © 2024 Answers in Genesis




